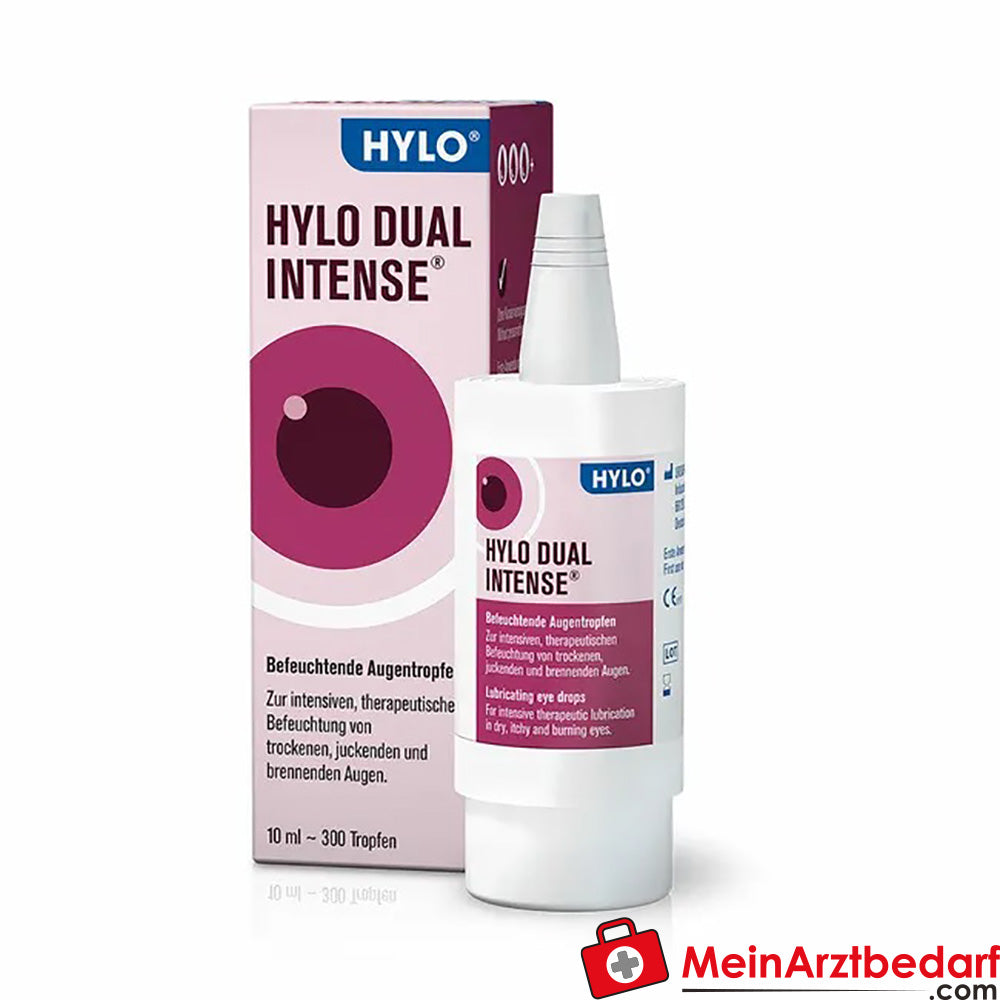
HYLO Dual Intense krople do oczu 0,2% kwas hialuronowy, 2,0% ektoina 10 ml
Marke: URSAPHARM Arzneimittel GmbH
W celu zapoznania się z ryzykiem i działaniami niepożądanymi należy przeczytać ulotkę dla pacjenta i zwrócić się do lekarza lub farmaceuty. (tekst obowiązkowy)
Verkauf & Versand durch: Lahn-Apotheke Marburg
Zakres dostawy
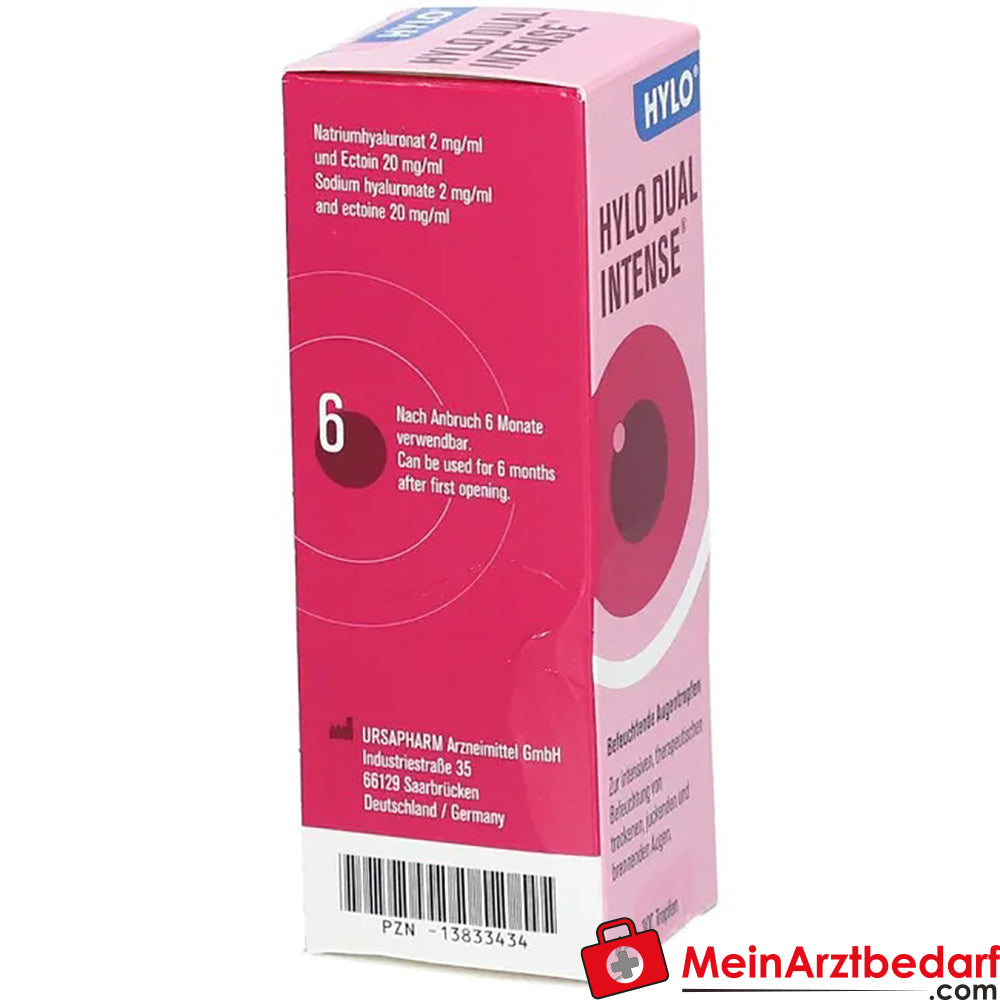
Zakres dostawy: 1 butelka kropli do oczu HYLO DUAL INTENSE 0,2% kwas hialuronowy / 2,0% ektoina, sterylne, 10 ml - Producent: Ursapharm GmbH
Szczegóły produktu i informacje obowiązkowe
HYLO DUAL INTENSE krople do oczu 0,2% kwasu hialuronowego / 2,0% ektoiny, 10 ml
Krople do oczu HYLO DUAL INTENSE zapewniają intensywne, długotrwałe nawilżenie i ochronę powierzchni oka w przypadku przewlekle suchych i podrażnionych oczu. Połączenie 0,2% kwasu hialuronowego i 2,0% ektoiny stabilizuje film łzowy, łagodzi podrażnienia i wspiera funkcję bariery bez konserwantów i fosforanów.
Stosowanie i instrukcje
Przed użyciem zdjąć nasadkę ochronną. Zakroplić jedną kroplę do worka spojówkowego; zamknąć oko na chwilę, aby rozprowadzić płyn. Zamknąć butelkę natychmiast po użyciu. Należy przeczytać ulotkę dołączoną do opakowania i zasięgnąć porady lekarza, jeśli objawy utrzymują się lub nasilają. W celu zapoznania się z ryzykiem i skutkami ubocznymi należy przeczytać ulotkę dołączoną do opakowania bądź skontaktować się z farmaceutą lub lekarzem.
Właściwości i specyfikacja
Dla kogo przeznaczony
Należy zapoznać się z ulotką dołączoną do opakowania i w razie wątpliwości skonsultować się z farmaceutą lub okulistą.
Ważne uwagi
Informacje na temat ryzyka i skutków ubocznych można znaleźć w ulotce dołączonej do opakowania oraz u lekarza lub farmaceuty.
Sprzedawca zastrzega sobie prawo do przeprowadzenia przez naszych farmaceutów kontroli farmaceutycznej ilości zamówionego leku. Może to spowodować zmniejszenie zamówienia na ten produkt. Kwota faktury zostanie wówczas automatycznie skorygowana.
Powodem tego jest nasz farmaceutyczny obowiązek opieki.

O MeinArztbedarf.com
Nasz sklep internetowy jest obsługiwany przez Mein Arztbedarf GmbH z siedzibą w Hall w austriackim Tyrolu. Specjalizujemy się w zaopatrzeniu medycznym, diagnostyce, medycynie oddechowej i farmaceutykach.
Do kogo skierowana jest oferta?
Nasze usługi skierowane są do profesjonalistów medycznych, instytucji i osób prywatnych.
Czy zamówienie jest bezpieczne?
Tak, korzystamy z szyfrowanego połączenia (SSL) i współpracujemy wyłącznie z certyfikowanymi dostawcami usług płatniczych.
Jakie metody płatności są dostępne?
Oferujemy płatności kartą kredytową, PayPal, Klarna, Sofortüberweisung, przedpłatę i zakup na konto.
Jak długo trwa wysyłka?
Z reguły wysyłka następuje w ciągu 1-3 dni roboczych, pod warunkiem, że towar znajduje się w magazynie. Więcej szczegółów można znaleźć bezpośrednio przy produkcie.
Do jakich krajów dostarczacie przesyłki?
Obecnie dostarczamy do Austrii, Niemiec, Włoch, Francji, Polski, Hiszpanii i Holandii.
Czy mogę zwrócić produkty?
Nieotwarte i nieużywane produkty można zwrócić w ciągu 14 dni - chyba że są to produkty higieniczne lub leki. Zobacz Cofnięcie.
Czy dostępne są rabaty ilościowe lub oferty dostosowane do indywidualnych potrzeb?
Tak, w przypadku większych zamówień lub obiektów z przyjemnością przygotujemy indywidualną ofertę. Skontaktuj się z nami!