1 product
filterCleartest® PSA semi-quantitative rapid test from 3 ng/ml for whole blood/serum/plasma 1 test
Cancer marker tests
Product types and test formats
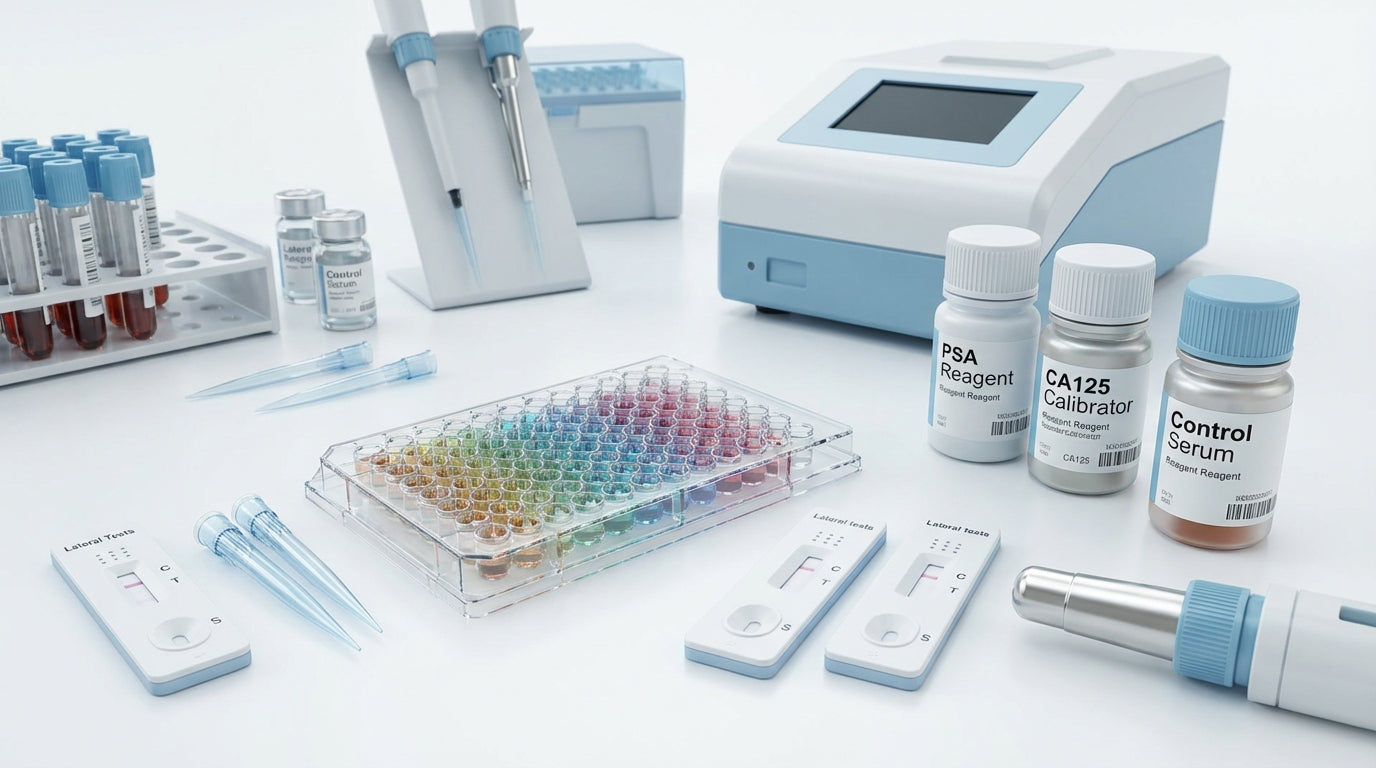
The category Cancer marker tests includes various test formats for laboratory and point-of-care detection of tumor markers. Typical product types are serological immunoassays (ELISA kits), rapid tests/lateral flow tests, automation reagents for analytical devices as well as calibrators and controls. Additional consumables such as sample tubes, pipette tips and reagent containers for routine laboratory organization complete the range.
- ELISA and chemiluminescence assays
- Rapid tests / lateral flow
- Calibrators, controls, reagents
- Sample preparation and consumables
Areas of application and typical application situations
Cancer marker tests are used in laboratory diagnostic monitoring, in screening scenarios for follow-up monitoring and in situation-dependent clarifications. In everyday life they are used for the quantitative determination of proteins such as PSA, CA125, CEA, AFP or CA19-9 in serum/plasma. The test solutions enable standardized processes: sampling, centrifugation, reagent preparation, measurement and quality control. Rapid tests also offer quick orientation results, while ELISA and automated systems provide precise laboratory values.
Quality, safety and organizational benefits
Products in the category are designed for reproducibility, batch control and easy integration into laboratory processes. Quality controls and calibrators support quality assurance; Validated reagents and standardized test kits make it easier to provide reliable results. Ergonomic packaging and pre-assembled test kits contribute to efficient sample processing and hygienic handling.
Brands and manufacturing environment
Solutions from manufacturers like Roche, Abbott, Siemens and Bio‑Rad can be found alongside specialized kit manufacturers and suppliers of consumables. The combination of established diagnostic systems and additional test kits allows both routine operation and project-related measurement tasks.
Important properties and selection criteria
When choosing Cancer marker tests Sensitivity, specificity, detection range, compatibility with existing analytical devices and available quality controls are decisive. Other decision factors include lead time, level of automation, storage and handling requirements, and availability of calibration materials.