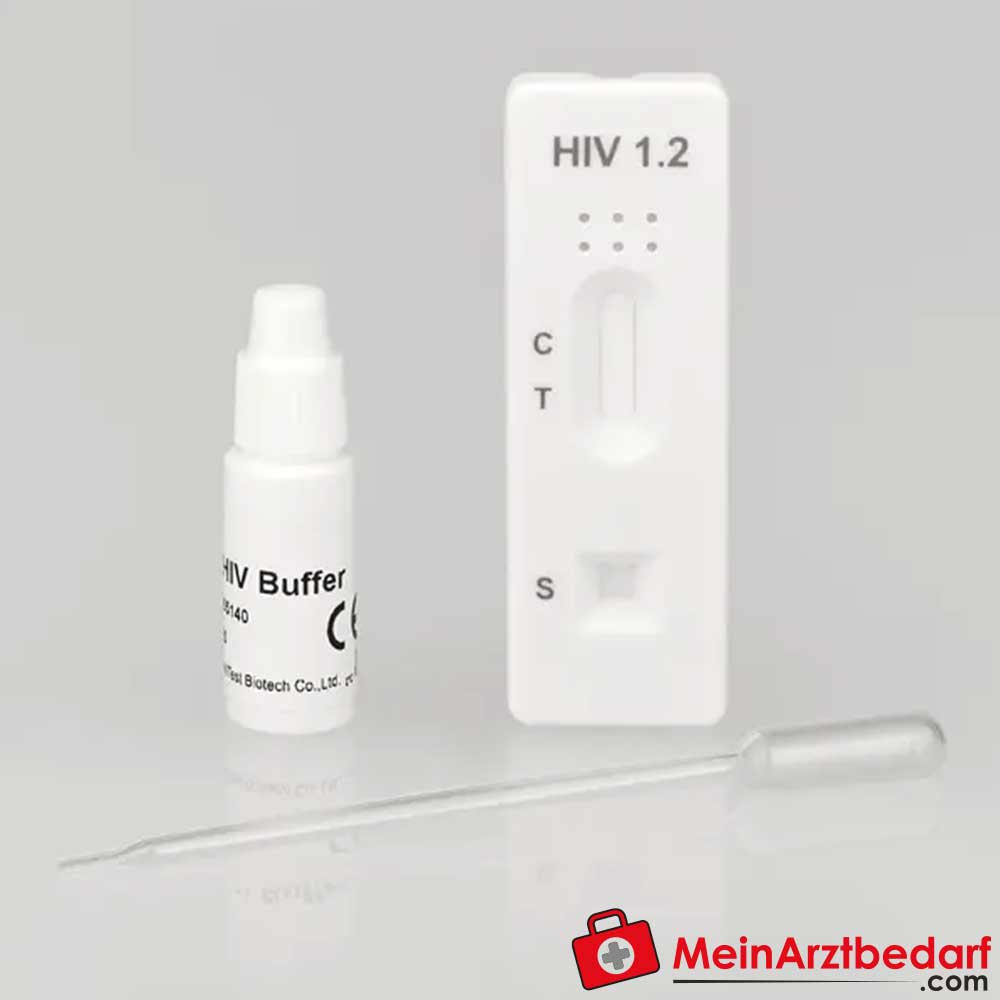
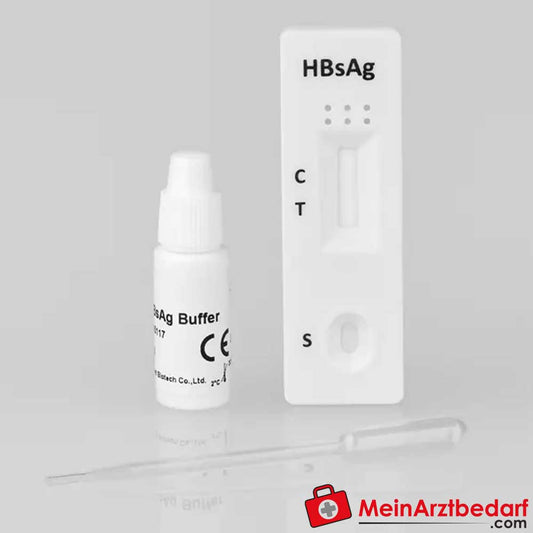
Cleartest HIV 1.2 Schnelltest für Vollblut/Serum/Plasma 10 St.
Marke: CLEARTEST
Zu Risiken und Nebenwirkungen lesen Sie die Packungsbeilage und fragen Sie Ihre Ärztin, Ihren Arzt oder in Ihrer Apotheke. (Pflichttext)
- Sofort lieferbar
Verkauf & Versand durch: Mein Arztbedarf GmbH
Lieferumfang
Lieferumfang: 1 Packung mit 10 Stück CLEARTEST® HIV 1.2 Schnelltest; Stechhilfe muss separat bestellt werden – Hersteller: Servoprax GmbH.
Produktdetails & Pflichtangaben
CLEARTEST® HIV 1.2 Schnelltest für Vollblut, Serum und Plasma – professioneller HIV-Antikörpernachweis (10 St.)
Der CLEARTEST® HIV 1.2 ist ein Schnelltest zum qualitativen Nachweis von Antikörpern gegen HIV‑1 und HIV‑2 in Vollblut, Serum oder Plasma. Entwickelt für die professionelle In-vitro-Diagnostik in Arztpraxen, Laboren und Einrichtungen . Schnelle, leicht ablesbare Ergebnisse zur Unterstützung diagnostischer Entscheidungen.
Anwendung & Hinweise
Zweckbestimmung: Qualitativer Nachweis von Antikörpern gegen HIV‑1 und HIV‑2. Durchführung nach Packungsanleitung; Ergebnisinterpretation durch farbige Linien. Für medizinisches Fachpersonal. Bei Unsicherheiten Packungsbeilage beachten oder ärztlichen Rat einholen.
Eigenschaften & Spezifikationen
Einsatzbereiche / Für wen geeignet
Hersteller: Servoprax GmbH
Wichtige Hinweise
Die Anwendung wird nur für medizinische Fachkräfte empfohlen
Hinweise zur Rückgabe
Für Verbraucher besteht das Widerrufsrecht nicht bei Verträgen zur Lieferung versiegelter Waren, die aus Gründen des Gesundheitsschutzes oder der Hygiene nicht zur Rückgabe geeignet sind, wenn ihre Versiegelung nach der Lieferung entfernt wurde.
Wichtige Hinweise
- Verwendung nur durch Fachpersonal: Der Test ist ausschließlich für die in-vitro Diagnostik durch geschultes Personal bestimmt.
- Lagerung: Der Test sollte bei Raumtemperatur oder gekühlt (2-30 °C) gelagert werden und ist bis zum auf der Verpackung angegebenen Verfallsdatum stabil. Nicht einfrieren.
- Probenhandhabung: Für präzise Ergebnisse muss der Test unmittelbar nach der Probenentnahme durchgeführt werden. Proben sollten nicht mehrfach eingefroren und aufgetaut werden.
Häufig zusammen gekauft

Über MeinArztbedarf.com
Unser Onlineshop wird von der Mein Arztbedarf GmbH mit Sitz in Hall in Tirol, Österreich betrieben. Wir sind spezialisiert auf medizinischen Fachbedarf, Diagnostik, Beatmungsmedizin und Arzneimittel.
An wen richtet sich das Angebot?
Unser Angebot richtet sich an medizinisches Fachpersonal, Institutionen und Privatpersonen.
Ist die Bestellung sicher?
Ja! Wir nutzen eine verschlüsselte Verbindung (SSL) und arbeiten ausschließlich mit zertifizierten Zahlungsanbietern.
Welche Zahlungsmethoden gibt es?
Wir bieten u. a. Kreditkarte, PayPal, Klarna, Sofortüberweisung, Vorkasse und Kauf auf Rechnung.
Wie lange dauert der Versand?
In der Regel erfolgt der Versand innerhalb von 1–3 Werktagen, sofern die Ware lagernd ist. Genaueres finden Sie direkt beim Produkt.
In welche Länder wird geliefert?
Wir liefern derzeit nach Österreich, Deutschland, Italien, Frankreich, Polen, Spanien und in die Niederlande.
Kann ich Produkte zurückgeben?
Ungeöffnete und unbenutzte Produkte können Sie innerhalb von 14 Tagen zurückgeben – außer es handelt sich um Hygiene- oder Arzneimittel. Siehe dazu den Widerruf.
Gibt es Mengenrabatte oder individuelle Angebote?
Ja! Für größere Bestellungen oder Einrichtungen erstellen wir gerne ein individuelles Angebot. Kontaktieren Sie uns!